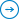In fifteen years of R&D management I have developed products and processes in both academic and corporate research environments. At every stage in my career and in every organization I have been a part of there have been an incredible number of oversight and compliance procedures required. This may be due to regulatory or financial controls, but the result is that the average initiation and execution of a research project involves the input and approval of many parties. This often requires an immense amount of time and effort to coordinate. As those involved in research administration know, efficient and expedient coordination of the various parties and tasks has always been an issue. As universities face more pressure to show the fruits of public funding and become more transparent with findings, the ability to clearly show handling of compliance procedures has grown in importance.
Corporations, too, are experiencing more complex approval chains. Global research networks, the rise of co-development, and complex R&D processes have increased the average number of approvals needed for a single project. Only last week I was trying to coordinate approval from six authors of a journal submission when three were no longer with the company. Wellspring has long been the leader in research and innovation software. We are proud to serve these markets and are excited to officially become a DocuSign Certified Solution. DocuSign is the global leader in digital transaction management and e-signature routing. Our partnership will reduce manual process errors and rekeying of data and eliminate the printing, scanning, and emailing of documents. No more overnighting of documents, faxing, or waiting—and it is more secure than paper.
Academic Approval Process
Competition for federal and industry-sponsored funding is at an all-time high. In fact, grant applications in 2015 are likely to set records. The NIH alone will award over 50,000 grants to 300,000+ researchers this year. While the motivation to access this funding has grown, the ability to do so efficiently has diminished. Looking at a common sample document, “Steps for Grant Routing Process,” you can see that a large number of signatures is required. Time lines for those approval steps vary, as do the department and location of the respective parties. Tracking down each individual’s approval while maintaining the required timeline is a essential to professional research operations. However, old-fashioned pen and paper can eat countless hours of otherwise productive time and make it difficult to visualize bottlenecks and the true sources of delays in the overall process. Once more, the researcher is likely to be pursuing multiple grants with multiple authorities simultaneously. The flow chart below offers a glimpse of the complexity researchers face when dealing with numerous types of approvals. You can see that approval chains and document routings are important steps in the process, but without sufficient tools for managing these steps digitally, document approvals can quickly become difficult and opaque.
Corporate Collaboration
These issues are not confined to academia. While universities may have to deal with approval chains stretching across campus, modern corporations conduct research on a global scale. Imagine the difficulty of routing project approvals from labs in India to managers in Spain to division heads back in the United States. Imagine the time wasted every year simply because you are chasing approvals halfway around the globe—approvals that, in many cases, need to be redone because of mistakes or missed signatures. Then factor in the growing rate of co-development in industrial research. The IRI 2015 Trends Forecast predicts collaborative research efforts to grow across the board, with 47 percent of respondents predicting an increase in joint venture and alliance research projects. Every new project will require another set of NDAs and CDAs and coordination between individuals at different firms. Sharing research activity means creating another layer of approval chains and required signatures, and dealing with the ensuing paperwork adds to a project’s timeline. Obviously, co-development is a good thing in the long run, but without a way to actively track research agreements and project details, efficiency will suffer.
On the Bright Side . . .
Some organizations have been able to take advantage of growing grant pools, global knowledge networks, and increased collaboration. The future of R&D is undoubtedly complex, and the administrative hurdles should not be ignored. With a dedicated process for tracking knowledge assets, monitoring portfolios, and routing approval signatures, research organizations can save countless hours and drive the next wave of innovation by spending more time on research and less on administration. Wellspring is proud to become a DocuSign Certified Solution and partner with the global leader in electronic document verification. By integrating the DocuSign envelope technology with our comprehensive knowledge supply chain software, Wellspring has created the first complete platform for modern research operations, allowing you to manage e-signatures and approval chains digitally.